Flying blind: Survey research among methadone and buprenorphine providers in Arizona
Research note
Brady, B., Meyerson, B.E. & Bentele, K. G. (2023). Flying blind: Survey research among methadone and buprenorphine providers in Arizona. Survey Methods: Insights from the Field. Retrieved from https://surveyinsights.org/?p=17985
© the authors 2023. This work is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0) 
Abstract
Survey studies of clinicians often face the challenge of achieving sufficient response rates to adequately understand practice realities. We briefly report our experience recruiting a sample of medication for opioid use disorder (MOUD) providers in a southwestern U.S. state (Arizona) to study the impact of temporary federal regulatory changes on their implementation of treatment accommodations to increase access to methadone and buprenorphine during COVID. After multiple and varied recruitment approaches using a modified Dillman method, we achieved a response rate of 5.4%. This was significantly lower than reported rates from other studies of 43-48%. To examine why we struggled to receive responses from those in our sampling frame, we matched our recruitment list with a curated, secret shopper MOUD provider calling list prepared in a separate Arizona study. Only 37% of providers on the DEA list were confirmed and located on the secret shopper list. We compared these lists to raise the question of whether the listings of MOUD providers by the Drug Enforcement Administration or Substance Abuse and Mental Health Administration are sufficient sources for practice-based survey recruitment. Locating and learning from MOUD providers is critical to understanding practice and patient outcomes for these lifesaving, but often difficult to access treatments for opioid use disorder.
Keywords
hybrid survey recruitment, medication for opioid use disorder, practice based research, QR codes, study recruitment, survey research
Funding for this study was provided by the Foundation for Opioid Response Efforts and the Vitalyst Health Foundation.
Copyright
© the authors 2023. This work is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0) 
Introduction
Survey studies of clinicians often face the challenge of achieving a sufficient response rate to accurately understand practice realities.[1-3] Survey participation barriers involve the lack of provider time, perceived relevance, and interruption of clinic or patient flow.[1,3,4] In prior studies of clinicians providing medication for opioid use disorders (MOUD), survey response rates have ranged from 43-48% for nurses, physician assistants and physicians.[5-7]
MOUD providers are a unique population of clinicians in the United States. Until very recently (December 29, 2022), they had to submit to the U.S. Drug Enforcement Administration (DEA) to receive a waiver to prescribe buprenorphine for the treatment of opioid use disorders (OUD). Many do not define themselves solely by this practice, as many treat an array of patient conditions that include but are not limited to OUD. It is also true that in the United States OUD is highly stigmatized[8] and patients experience significant treatment access barriers.[9,10] Thus, it is essential to understand clinician perspectives, MOUD practices, and related barriers in order to assure access to these lifesaving medications.
We briefly report our experience recruiting a state-wide sample of MOUD providers in a southwestern U.S. state (Arizona) to study the implementation of temporary federal regulatory changes which allowed flexibility in treatment accommodations to increase patient access to methadone and buprenorphine while reducing COVID exposure. Recruitment was similar to a Dillman method[11] with a paper invitation for an online survey while including a QR (quick response) code and a URL. This recruitment method was previously used among pharmacists and nurses, [12-14] with an invitation letter containing a 4-digit pass code pre-populating the providers’ name and address at the time of survey administration for confirmation. The survey contained 52 questions and was expected to take less than 30 minutes to complete. Providers completing the survey were offered an online Visa gift card valued at $20.00. Human subjects oversight was provided by the University of Arizona Institutional Review Board.
We briefly describe our multiple recruitment efforts and their limited outcomes. To contextualize the low response rate, we also report findings from a separate Arizona study in which we curated a list of confirmed MOUD providers whom we contacted through a secret shopper audit to gain a sense of how active DEA-listed MOUD providers are in offering MOUD services. We hope to engage readers and other practice-based survey researchers to collectively consider implications for future practice-based survey research and identify future approaches to mitigate some of these challenges.
Initial Recruitment Methods
In March 2021, we curated a list of Arizona MOUD providers using a DEA-provided listing of all waiver-holding clinicians approved to prescribe buprenorphine (n=2,320). Providers who were included on this list had previously received a DEA license to prescribe controlled substances and received a waiver to prescribe schedule III (buprenorphine) controlled substances to treat OUD. To distinguish providers who worked at methadone clinics (or OTPs: opioid treatment programs), we identified OTP practice addresses from the federal Substance Abuse and Mental Health Services Administration (SAMHSA) public Opioid Treatment Directory[15] and matched them with addresses on the DEA list. After geo-coding provider practice addresses, we removed 57 providers located in census tracks associated with federally recognized tribal governments because they were engaged in a separate process to understand MOUD treatment challenges in their communities.
The DEA list included provider classification (physician, physician assistant, nurse practitioner) and approved patient prescribing limits (30, 100, 275). We then coded provider practice locations by the four-category classifications by the University of Washington’s Rural Health Research Centre.[16]
Our original approach was a stratified random sampling of 1000 providers as a representative subset of all DEA providers. This comprised a census of all OTP-located providers and all non-urban providers, followed by a random sampling of non-rural buprenorphine providers. Three recruitment waves were implemented with an initial invitation followed by two successive postal mailed letter invitations to nonrespondents after 3-week periods.
Recruitment Experience
The MOUD provider survey based on the original random sample of 1000 providers was first fielded on September 14, 2021. Following the third recruitment wave, we received a total of 49 survey responses for a response rate of 4.9%. Our response rates consider partial interviews as respondents; they are Response Rate 2 as defined by the American Association for Public Opinion Research[17]. To improve our recruitment, we consulted opioid treatment coalitions for guidance. Partners at Arizona’s state-wide MOUD ECHO, a MOUD treatment learning collaborative of MOUD providers convened by the Arizona State University, advised that we also fax the invitation letter for a 4th wave of recruitment. The DEA list did not provide fax numbers, but this information was publicly available for the portion of providers who elected to be included on SAMHSA’s buprenorphine practitioner locator site.[18] This site provides public access to datafiles that contain providers’ names, practice addresses, and contact information, organized by state. To supplement, we conducted a manual internet search to locate fax numbers for remaining providers. Ultimately, we obtained fax information for 403 providers and began faxing as we located their information from November 17, 2021, to January 24, 2022. From the faxes, we received 12 additional survey responses.
During this period, we also discussed provider recruitment challenges with the Arizona state-wide Drug Policy Research and Advocacy Board (DPRAB), an interdisciplinary group of MOUD providers, harm reduction organizations, and people with lived substance use experience convened by the University of Arizona Harm Reduction Research Lab. The DPRAB recommended that we open the survey to social recruitment and reach out to associations of MOUD providers to assist.
To account for the overlap of recruitment methods, we created a mirrored survey location to separate the responses from faxes sent to the random stratified sample and those who participated from social recruitment efforts, and asked respondents to provide their name and practice information. This allowed us to compare differences in response by recruitment method. On December 15th, for the social recruitment, we sent invitation emails to organizations, associations, and coalitions that included Arizona MOUD providers. We asked them to forward our survey invitation to their members as an email attachment and provided email text for the cover communication. As with the mailed and faxed invitations, the emailed PDF attachment also contained a QR code and URL to the online survey. We were advised to send only one wave of organizational recruitment communications. The social recruitment method resulted in an additional 17 provider surveys. As the social recruitment could have captured respondents who were not waived providers, we examined these surveys and were able to link 15 responses to providers on the DEA list. These 15 were included in the study. The two responses that were not matched were not included as we could not confirm that they held an x-waiver or were approved by the DEA to provide MOUD. These 15 providers may have been but were not necessarily included in the original random sample of 1000.
Given the continued low response rate, a final decision was made to solicit all providers included on the original DEA list. This represented our census survey sampling frame. Removing prior responders and those located on tribal reservations, it included 2,261 providers from the DEA list. This constituted a fifth wave of recruitment that included all non-responders from the original 1,000 randomly sampled providers and a first and second wave for providers on the DEA list who were not included in the original random sample. We elected to use the original postal mail method and not the fax method. Based on resources and time, we conducted two waves of recruitment separated by three weeks. This began on January 28, 2022, with a second wave on February 18, 2022. On April 22, 2022, the survey was closed.
Across all methods of recruitment, we received a total of 122 survey responses achieving a 5.4% response rate from the pool of 2,264 providers on the curated DEA list. We observed response rate differences based on provider rurality and DEA-allowed MOUD patient panel size. Rurally located providers and those waived to prescribe MOUD to up to 275 patients responded at over twice the rate compared to urban providers (14.2% vs. 4.6%) and those with 30 or 100 patient limits (11.6% vs. 4.9%). Table 1 displays responses by respondent characteristics for each sampling group.
Table 1. MOUD* Provider Survey Response Rate by Method of Recruitment (N=122), Arizona 2022
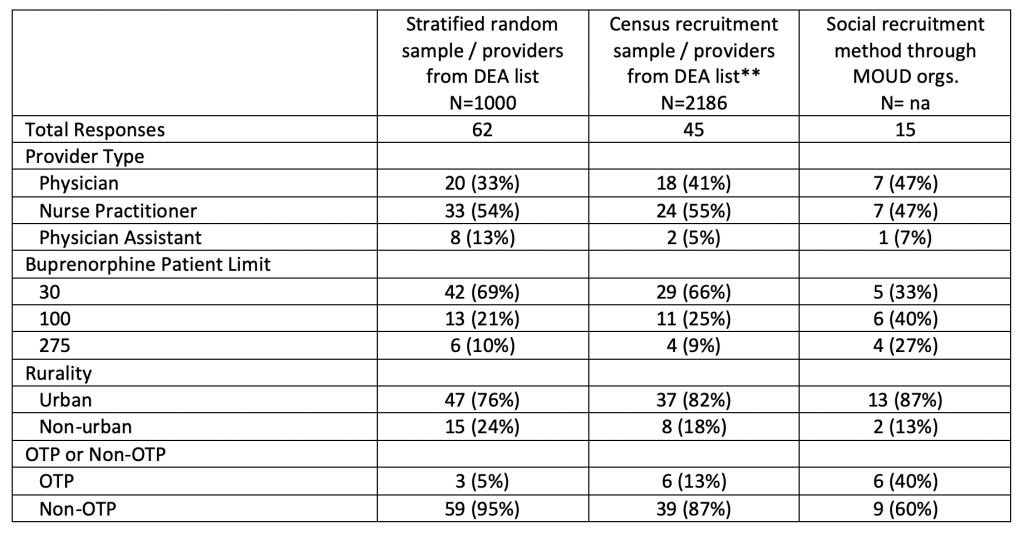
*MOUD: medication for opioid use disorder
**Census recruitment sample included all providers on the DEA list (2,320) with removal of providers on tribal lands (57), those reached as part of the initial stratified random sampling effort (62), and those reached through social recruitment (15).
Subtotals may not sum to count of total responses due to missing data.
During the period of provider survey response and prior to the social recruitment method, the DPRAB called for a secret shopper study to call MOUD providers. The goal was to better understand whether providers on the DEA list were active MOUD providers, if they were accepting new patients, or if they implemented COVID regulatory changes. This process and results will be reported elsewhere;[19] however, to provide context for recruitment in this study, we report the final count of providers on the curated secret shopper call list and our match with it. Ascertaining if providers are active MOUD prescribers offers insight into the value of using the DEA list in deriving a sampling frame.
The secret shopper list of MOUD providers was developed using the original DEA list. To identify which of the original DEA-listed providers to include in the secret shopper audit, we conducted an internet search of names and addresses and manually removed those in specialties or likely not providing treatment for OUD (e.g., anaesthesiology, surgery, et cetera) and those who could not be located or whose address was not matched with a phone number through internet search. Inclusion on the secret shopper list was intended to reflect the conditions of outpatient MOUD treatment. If a person was not in a practice setting where an individual would normally look for OUD treatment or did not have publicly available practice information or contact information, they were not included on the secret shopper list. The resulting list included 838 providers, or 37% of the providers from the original DEA list.
In Table 2, we compare demographic differences between the original DEA list, those reached for survey, and the those on the secret shopper list. Provider type, patient limit, rurality, and OTP status were similar between the DEA and the secret shopper list. However, there were substantial differences between these groups and the providers who participated in the COVID MOUD study. Comparatively, a higher proportion of study participants were nurse practitioners, had 100 and 275 buprenorphine patient limits, and worked in non-urban areas and OTPs.
Table 2. MOUD* Provider Survey Response Rate by Method of Recruitment (N=122), Arizona 2022
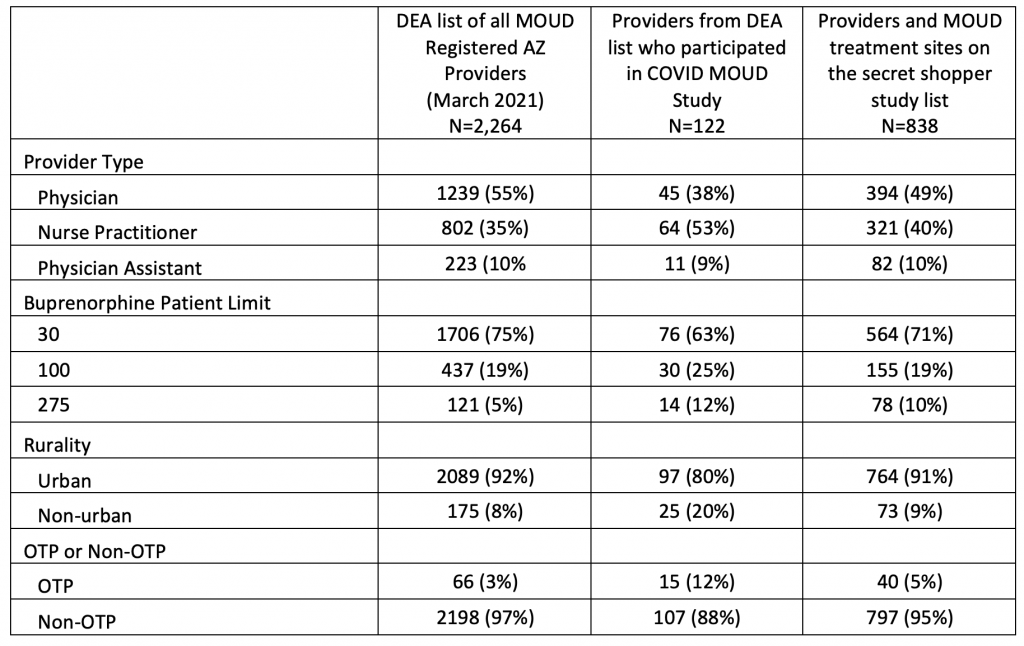
*MOUD: medication for opioid use disorder
Subtotals may not sum to count of total responses due to missing data.
Discussion
Findings reported here suggest that multiple and distinct contact attempts with Arizona MOUD providers using government-provided listings will result in insufficient samples for methadone and buprenorphine practice-based research. When deriving a sampling frame, it is important that the list of potential participants is relevant to the research topic and reachable with up-to-date contact information. In our effort to reach Arizona MOUD providers, the DEA list was not useful for this purpose. In theory, the list provides the names and addresses of those who intend to prescribe MOUD. However, our findings suggest that the list is not sufficiently specific to inform the denominator for survey-based research among MOUD providers.
It is possible that the purpose of the DEA list is overly broad, such that its sensitivity and specificity is ill suited for deriving a MOUD-focused research sample. For example, providers may request a waiver to prescribe buprenorphine for OUD treatment and be included on the DEA list for reasons unrelated to their intention to treat this patient population. They may have applied for a waiver as part of residency program requirements or to receive preference for a loan-forgiveness award[20], without the intention of consistently prescribing MOUD in their professional practice. It may be problematic to assume that DEA-listed providers are active or current MOUD providers in a state or jurisdiction.
Prior researchers have used the DEA list to assess MOUD providers’ geographic distribution[21,22], and to assess prescribing activity[23]. For example, Andrilla, Coultard, and Patterson used the DEA list to survey all US, rurally located providers about their past and current MOUD practices. Similar to our experience, they found errors in the DEA file. After manually updating provider contact information, their research team used a combination of up to 3 letters, 1 postcard, and 3 phone calls to reach 60.5% of their target sample, a higher response rate than the 5.4% we achieved. In our sample, we reached more rural than urban providers, so the difference may be partially explained by rural providers being more responsive. However, Andrilla et al.’s survey approach also differed from ours. They corrected addresses by matching and pulling information from other datasets and through Google searches and they called every provider that didn’t respond to mailed letters.
Another important factor that may affect response rates relates to the length and focus of the survey. Andrilla et al.’s survey included 15 items compared to our 52-item instrument. Shorter surveys have higher participation rates.[24,25] Andrilla et al.’s survey also differed in its purpose; it focused on providers’ history and current MOUD prescribing activity. Those not actively treating MOUD patients may be willing to say as much in a short survey. Almost half of Andrilla et al.’s respondents reported that they were not currently accepting new MOUD patients, a similar proportion also found in a US study of prescription data[26]. Our study focused on providers’ experience related to implementing MOUD policies. Asking our participants to share their knowledge and experience in implementing MOUD policy changes during COVID presumes that they identify as providers for whom these policies are relevant. Based on extant literature, this may be true for only half of our sampling frame. When we sought to identify providers to include in a secret shopper audit study, we concluded with a list less than half the size of the original DEA list. The DEA list appears to provide a poor sampling frame for MOUD policy and practice research as it appears to include a substantial number of individuals who do not identify as MOUD providers.
Clinicians are a population that is normally busy, extra-stressed from COVID, and located across a range of practice settings. While this is widely recognized, we expected and planned for a response rate of around 30%, even though some studies (noted above) received response rates in the 40% range among MOUD clinicians. As found, only 37% of the providers in our census sample matched a carefully curated list for secret shopper inclusion. When we asked providers on the DPRAB and on the MOUD ECHO their thoughts about the low response rates, their hypotheses involved time constraints, the impact of COVID, and that letters may not have reached providers (not forwarded by office staff). Johnston and colleagues found something similar, where office staff did not forward the survey invitation to clinicians.[3] Unlike survey studies recruiting clinicians based on their primary specializations, in office-based settings, prescribing MOUD is a sub-specialty service offered to only a minority of patients. Among competing priorities, our request to answer this survey may not have been deemed as important as other tasks. Our survey results provide some support for this because providers waived to treat more patients for OUD responded at higher rates than those with a lower patient limit.
The DEA’s MOUD waiver list was discontinued in January 2023 due to changes in US Federal policy that removed the waiver requirement to prescribe MOUD. This introduces new questions about how to identify and sample providers to study access to and the quality of MOUD services. It also leaves unaddressed questions around how government-provided lists can supply accurate information for MOUD service locator tools, like SAMHSA’s buprenorphine practitioner locator[18]. Future research is needed to address these questions. Doing so will assist researchers to accurately draw sampling frames and for community members to correctly locate treatment sites.
To improve access and increase the quality of OUD treatment, we must identify and communicate with MOUD providers of this lifesaving service. Although MOUD reduces risk of overdose, bloodborne infections, recidivism, and all-cause mortality,[23] only about 28% of people with an opioid use disorder (OUD) receive treatment.[27] Unfortunately, the need for MOUD is currently outpacing increases in MOUD services.[28,29] Solving the challenge of MOUD provider research recruitment is a timely problem that requires our attention, especially now as policy leaders are considering additional federal and policy changes to treatment access, especially in the methadone treatment space. If not, our sampling will continue to be based on convenience and will not represent the true picture of MOUD access or treatment practice.
References
[1] Asch, S., Connor, S.E., Hamilton, E.G., Fox, S.A. (2000). Problems in recruiting community-based physicians for health services research. J Gen Intern Med, 15:591-599.
[2]Levinson, W., Dull, V.T., Roter, D.L., Chaumeton, N., Frankel, R.M. (1998). Recruiting physicians for office-based research. Med Care, 36:934-937.
[3]Johnston, S., Liddy, C., Hogg, W., Donskov, M., Russell, G., Gyorfi-Dyke, E. (2010). Barriers and facilitators to recruitment of physicians and practices for primary care health services research at on center. BMC Res Method, 10:109-116.
[4] Ross, S., Grant, A., Counsell, C., Gillespie, W, Russell, I., Prescott, R. (1999). Barriers to participation in randomised controlled trials: a systematic review. J Clin. Epidemiol, 52:1143-1156.
[5] Andrilla, C., Jones, K.C., Patterson, D.G. (2020). Prescribing practices of nurse practitioners and physician assistants waivered to prescribe Buprenorphine and the barriers they experience prescribing buprenorphine. J Rural Health, 36(2):187-195.
[6] Wisniewski, A.M., Dlugosz, M.R., Blondell, R.D. (2013). Reimbursement and practice policies among providers of Buprenorphine-Naloxone treatment. Substance Abuse, 34(2):105-107.
[7] Kermack, A., Flannery, M., Tofighi, B., McNeely, J., Lee, J.D. (2017). Buprenorphine prescribing practice trends and attitudes among New York providers. J Substand Abuse Treatment, 74:1-6.
[8]Olsen, Y., Sharfstein, J.M. (2014) Confronting the Stigma of Opioid Use Disorder—and Its Treatment. JAMA, 311(14):1393–1394.
[9] Lister, J.J., Weaver, A., Ellis, J.D., Himle, J.A., Ledgerwood, D.M. (2020). A systematic review of rural-specific barriers to medication treatment for opioid use disorder in the United States, American J Drug and Alcohol Abuse, 46:3, 273-288
[10] Wakeman, S.E., Rich, J.D. (2018). Barriers to Medications for Addiction Treatment: How Stigma Kills, Substance Use Misuse, 53:2, 330-333
[11] Dillman, D.A. (1978). Mail and telephone surveys: the total design method (New York: John Wiley & Sons).
[12] Agley, J.D., Meyerson, B.E., Eldridge, L.A., Jun, M., Vadiei, N., Crosby, R.A., Bentele, K.G., Kennedy, A., Anderson, K. (2022). Exploration of pharmacist comfort with harm reduction behaviors: Cross-sectional latent class analysis. J Am Pharmacists Assoc, 62(2):432-440.
[13] Carter, G.A., Meyerson, B.E., Rivers, P., Crosby, R.A., Lawrence, C.A., Cope, S.D., DeBruicker, D., Levin, S., Meeks, W., Thomas, C., Turner, B., Abert, C., Coles, H.B., Allen, A., Gonzales-Fagoaga, E., Grivois-Shah, R. (2021) Living at the confluence of stigmas: PrEP awareness and feasibility among people who inject drugs in two predominantly rural states. AIDS Behavior 2021; 25: 3085-3096.
[14] Meyerson, B.E., Agley, J.D., Jayawardene, W., Eldridge, L.A., Arora, P., Smith, C., Vadiei, N., Kennedy, A., Moehling, T., and the PharmNet Research Team. (2020). Feasibility of a pharmacy-based harm reduction intervention to reduce opioid overdose, HIV and hepatitis C – Indiana, 2019. Res Soc Admin Pharm, 16(5):699-709.
[15] United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration. Opioid Treatment Program Directory. [online]: https://dpt2.samhsa.gov/treatment/directory.aspx accessed August 9, 2022.
[16] University of Washington, Rural Health Research Center. Map Classifications. [online]: https://depts.washington.edu/uwruca/ruca-maps.php Accessed August 25, 2022.
[17] The American Association for Public Opinion Research. 2023 Standard Definitions:
Final Dispositions of Case Codes and Outcome Rates for Surveys. 10th edition. AAPOR
[18] U.S. Substance Abuse and Mental Health Services Administration. Medication Assistant Treatment Practitioner Locator. [online]: https://www.samhsa.gov/medication-assisted-treatment/find-treatment/treatment-practitioner-locator Accessed August 25, 2022.
[19] Meyerson BE, Treiber D, et al. Working paper from the MOUD provider secret shopper study.
[20] U.S. Health Resources and Services Administsration. National Health Service Corps (NHSC) Substance Use Disorder Workforce Loan Repayment Program. [online]: https://nhsc.hrsa.gov/loan-repayment/nhsc-sud-workforce-loan-repayment-program Accessed May 8, 2023.
[21] Andrilla CHA, Moore TE, Patterson DG, Larson EH. Geographic Distribution of Providers With a DEA Waiver to Prescribe Buprenorphine for the Treatment of Opioid Use Disorder: A 5-Year Update. The Journal of Rural Health. 2019;35(1):108-112. doi:10.1111/jrh.12307
[22] Andrilla CHA, Patterson DG. Tracking the geographic distribution and growth of clinicians with a DEA waiver to prescribe buprenorphine to treat opioid use disorder. The Journal of Rural Health. 2022;38(1):87-92. doi:10.1111/jrh.12569
[23] Andrilla CHA, Coulthard C, Patterson DG. Prescribing Practices of Rural Physicians Waivered to Prescribe Buprenorphine. American Journal of Preventive Medicine. 2018;54(6, Supplement 3):S208-S214. doi:10.1016/j.amepre.2018.02.006
[24] Haffajee, R.L., Lin, L.A., Bohnert, A.S.B., Goldstick, J.E. (2019). Characteristics of US Counties With High Opioid Overdose Mortality and Low Capacity to Deliver Medications for Opioid Use Disorder. JAMA Netw Open, 2(6):e196373. doi:10.1001/jamanetworkopen.2019.6373
[25] Rolstad S, Adler J, Rydén A. Response burden and questionnaire length: Is shorter better? A review and meta-analysis. Value in Health 2011; 14(8):1101-1108.
[26] Kost RG, de Rosa JC. Impact of survey length and compensation on validity, reliability, and sample characteristics for Ultrashort-, Short-, and Long-Research Participant Perception Surveys. J Clin Transl Sci. 2018 Feb;2(1):31-37. doi: 10.1017/cts.2018.18.
[27] Duncan A, Anderman J, Deseran T, Reynolds I, Stein BD. Monthly Patient Volumes of Buprenorphine-Waivered Clinicians in the US. JAMA Network Open. 2020;3(8):e2014045. doi:10.1001/jamanetworkopen.2020.14045
[28] Mauro, P.M., Gutkind, S., Annunziato, E.M., Samples, H. (2022). Use of Medication for Opioid Use Disorder Among US Adolescents and Adults With Need for Opioid Treatment, 2019. JAMA Netw Open, 5(3):e223821.
[29] Krawczyk, N., Rivera, B.D., Jent, V., Keyes, K.M., Jones, C.M., Cerdá, M. (2022). Has the treatment gap for opioid use disorder narrowed in the U.S.?: A yearly assessment from 2010 to 2019″. International Journal of Drug Policy. doi.org/10.1016/j.drugpo.2022.103786





